
Each group around the central atom is designated as a bonding pair (BP) or lone (nonbonding) pair (LP). In the VSEPR model, the molecule or polyatomic ion is given an AX mE n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group (usually a lone pair of electrons), and m and n are integers. That is, the one that minimizes repulsions. Groups are placed around the central atom in a way that produces a molecular structure with the lowest energy. Groups are positioned around the central atom in a way that produces the molecular structure with the lowest energy, as illustrated in Figure 9.1 "Common Structures for Molecules and Polyatomic Ions That Consist of a Central Atom Bonded to Two or Three Other Atoms" and Figure 9.2 "Geometries for Species with Two to Six Electron Groups".įigure 9.2 Geometries for Species with Two to Six Electron Groups Because electrons repel each other electrostatically, the most stable arrangement of electron groups (i.e., the one with the lowest energy) is the one that minimizes repulsions. According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present. Lewis electron structures predict the number and types of bonds, whereas VSEPR can predict the shapes of many molecules and polyatomic ions. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality the model provides no information about bond lengths or the presence of multiple bonds.

(pronounced “vesper”), which can be used to predict the shapes of many molecules and polyatomic ions. We continue our discussion of structure and bonding by introducing the valence-shell electron-pair repulsion (VSEPR) model A model used to predict the shapes of many molecules and polyatomic ions, based on the idea that the lowest-energy arrangement for a compound is the one in which its electron pairs (bonding and nonbonding) are as far apart as possible.
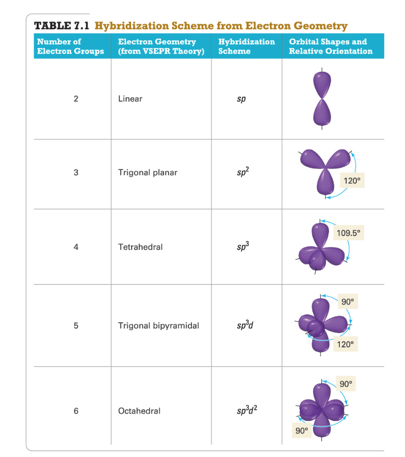
This approach gives no information about the actual arrangement of atoms in space, however. The Lewis electron-pair approach described in Chapter 8 "Ionic versus Covalent Bonding" can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. To predict whether a molecule has a dipole moment.To use the VSEPR model to predict molecular geometries.You will also discover why carbon, the basic component of all organic compounds, forms four bonds despite having only two unpaired electrons in its valence electron configuration and how the structure of retinal, the key light-sensing component in our eyes, allows us to detect visible light. The tools you acquire in this chapter will enable you to explain why Ca 2 is too unstable to exist in nature and why the unpaired electrons on O 2 are crucial to the existence of life as we know it. We conclude by describing more complex molecules and ions with multiple bonds. We apply two distinct approaches for describing covalent bonds: (1) a localized model to describe bonding in molecules with two or more atoms attached to a central atom and (2) a delocalized model to explain and predict which diatomic species exist and which do not exist. In this chapter, we begin with a general method for predicting the structures of simple covalent molecules and polyatomic ions then we discuss the actual distribution of electrons in covalent bonds. This image shows that the bonding electrons on the copper atom in Cu 2O occupy d z 2 orbitals that point toward the oxygen atoms located at the center and corners of a cube.
An experimental image of a covalent bond.


 0 kommentar(er)
0 kommentar(er)
